What is Chloramphenicol CAS 56-75-7?
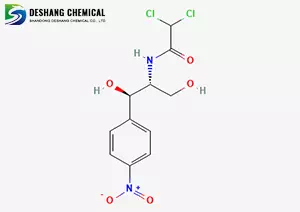
Molecular formula and structure
Molecular formula: C₁₁H₁₂Cl₂N₂O₅
Molecular weight: 323.13 g/mol
Structure: It contains p-nitrophenyl, propylene glycol and dichloroacetamide groups, and has a chiral center (the 1R and 2R configurations are the most active).
Appearance: White to slightly yellowish-green needle-like crystals or powder, extremely bitter in taste, and prone to yellowing under light (should be stored away from light).
Chloramphenicol (CAS 56-75-7), as the first fully synthetic antibiotic, has both core values and risks:
Irreplaceable clinical status: The ultimate choice for meningitis/typhoid fever, especially for those allergic to penicillin;
Prominent safety risks: Aplastic anemia and gray baby syndrome limit its application.
Process iteration direction: Enzymatic synthesis reduces environmental toxicity, and chiral purification enhances therapeutic efficacy.
Chloramphenicol CAS 56-75-7 Use
Pharmacokinetics
Absorption: Oral bioavailability is 75-90%, reaching peak within 0.5-2 hours (oral 1g blood drug concentration is 8-10 mg/L).
Distribution: Cerebrospinal fluid concentration ≈ 50% of blood drug concentration (up to 90% in meningitis), can penetrate the placenta.
Metabolism/Excretion: Inactivated by glucuronic acid in the liver, 90% of the metabolites are excreted through the kidneys, with a half-life of 2.5 hours (extended to 24 hours in newborns).
Chloramphenicol CAS 56-75-7 safety
1. Serious adverse reactions
● Bone marrow suppression
○ Reversible cytopenia (dose-dependent).
○ Aplastic anemia (incidence rate 1:30,000, mortality rate 50%) is dose-independent, and its mechanism is the inhibition of mitochondrial protein synthesis in the host.
● Gray baby syndrome: Due to insufficient liver enzymes in newborns, drug accumulation occurs, resulting in cyanosis and circulatory failure (contraindicated for premature infants and infants under 2 weeks old).
● Neurotoxicity: High doses can cause confusion and epilepsy (high risk in patients with renal insufficiency).
2. Contraindications and caution:
● Absolute contraindications: Late pregnancy, lactation period, history of bone marrow diseases, and patients with mental illness.
● Drug interactions
○ Antagonize the bactericidal effect of penicillin and prohibit the use of combined regimens.
○ Inhibit liver enzymes and increase the blood drug concentrations of phenytoin and warfarin.
Service
* Prompt reply and 24 hours online, professional team to provide best price and high quality product.
* Sample testing support.
* Every batch of products will be tested to ensureits quality.
*The packing also can be according the customers` requirment.
*Any inquiries will be replied within 24 hours.
*we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate. If your markets have any special requirements, let us know.