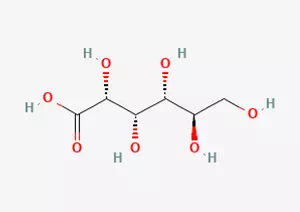
In the vast field of organic chemistry, gluconic acid, as an organic acid of great significance, has the molecular formula C6H12O7. This unique molecular formula determines a series of special properties it exhibits in terms of chemical reactions and material characteristics.
From the perspective of molecular structure, gluconic acid contains a hydroxylated straight chain composed of five carbon atoms. This hydroxylated straight chain is like a carefully constructed chain, and the hydroxyl group (-OH) on each carbon atom is like a specific decoration on the chain, endowing the entire molecule with specific chemical activity and polarity. Closely connected to this hydroxylated straight chain is a carboxylic acid group (-COOH). The presence of carboxylic acid groups endows gluconic acid with acidic chemical properties, enabling it to undergo acid-base reactions under certain conditions.
When the pH value of the environment is greater than 4, gluconic acid will undergo a deprotonation reaction, thereby generating the corresponding gluconate. This process is like a subtle chemical transformation. Under specific acidic and alkaline conditions, the hydrogen ions (H⁺) in the gluconic acid molecule will be released, causing changes in the charge distribution and chemical properties of the entire molecule, and then forming gluconate. This property has significant application value in many chemical analyses and industrial production processes.
Gluconic acid is a natural component that is widely present in nature. In the world of microorganisms, gluconic acid may be present in all kinds of tiny life forms. They might be the products of the metabolic processes of certain microorganisms, or they could be part of the structure of microbial cells. Similarly, among the rich variety of vegetables and fruits, gluconic acid also exists quietly. During the process of growth, development and metabolism, these vegetables and fruits will synthesize gluconic acid through their own physiological mechanisms. For instance, during the ripening process of some fruits, gluconic acid may be involved in processes such as the formation of fruit flavor and texture changes.
Not only that, gluconic acid is also present in honey and wine consumed by humans. Among these delicious foods, glucose is oxidized to form gluconic acid under the action of catalase. Catalase is like a precise "chemical craftsman". It can catalyze the reaction between glucose and oxygen, promoting the oxidation of glucose and ultimately converting it into gluconic acid. This natural oxidation process not only adds unique flavors and textures to honey and wine, but also provides people with a way to take in gluconic acid.
Interestingly, the same mode of action has also been ingeniously applied to the industrial production of microbial fermentation of gluconic acid. In large-scale industrial production environments, people take advantage of the fermentation capacity of microorganisms. By precisely controlling fermentation conditions such as temperature, pH value, and oxygen supply, microorganisms multiply in large numbers and act on starch-rich raw materials, thereby efficiently converting glucose into gluconic acid under the catalysis of catalase.
In the production process of gluconic acid, its raw materials mainly come from starchy grains, such as common wheat, barley, corn, etc., which are all high-quality raw material sources. First of all, starch slurry needs to be obtained from these grains. This process usually involves a series of complex operations such as cleaning, soaking and grinding the grains to ensure that the starch can be fully released from the grains and form a uniform starch slurry.
After obtaining the starch slurry, the next step is to carry out enzymatic hydrolysis treatment. During this process, specific enzymes are added to the starch slurry. These enzymes act like a pair of precise "chemical scissors", capable of breaking down starch molecules into free glucose molecules. Through enzymatic hydrolysis, the macromolecular substance starch is gradually decomposed, and eventually free glucose is obtained, preparing for the subsequent fermentation process.
Subsequently, free glucose will be converted into gluconic acid through fermentation. In the fermentation tank, microorganisms start their "work" under suitable conditions, converting glucose into gluconic acid. This process requires strict control of fermentation conditions, including temperature, pH value, oxygen content, etc., to ensure that the fermentation reaction can proceed smoothly and obtain high-yield and high-quality gluconic acid.
After the fermentation is completed, the product still needs to be purified. Through a series of physical and chemical methods, impurities and by-products in the fermentation broth are removed, and gluconic acid is purified to a sugar-water ratio of 50/50. This purified gluconic acid can be used for commercial sales to meet the demands of various industries. Alternatively, depending on specific application scenarios and market demands, gluconic acid can be neutralized into gluconate, further expanding its application scope.