What is Trichloroacetic acid CAS 76-03-9?
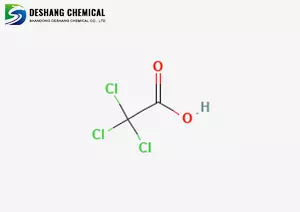
Molecular formula and structure
Molecular formula: C₂HCl₃O₂
Molecular weight: 163.39 g/mol
Structure: In the acetic acid molecule, the three hydrogen atoms of the methyl group are replaced by chlorine (Cl₃, CCOOH), making it a strong organic acid.
Appearance: Colorless to white crystals, hygroscopic, with a pungent odor.
It decomposes into chloroform and carbonate when exposed to strong alkali. Toxic gases such as hydrogen chloride and carbon monoxide are released at high temperatures.
Dilute solutions (<30%) are prone to decomposition and should be prepared and used immediately.
Trichloroacetic acid CAS 76-03-9 Use
1.Biochemistry and Medicine (accounting for 45%)
Protein precipitant
The precipitation of proteins through acid denaturation and exposure of hydrophobic groups is used to extract biochemical drugs such as ATP and cytochrome C.
Note: Macromolecular proteins (>100 kDa) may produce fragments due to acid hydrolysis, and the residues should be thoroughly washed off with acetone.
Pharmaceutical application
Dermatological wart remover (concentration 80-90%), astringent and hemostatic; Sodium trichloroacetate is a selective herbicide.
2. Agriculture and Industry (accounting for 35%
Pesticide intermediates: Key raw materials for the synthesis of the insecticide chlorpyrifos and the herbicide sulfoxpiazole.
Industrial Solvents and Synthesis
Introducing chlorine atoms in dye/pigment synthesis enhances stability;
Metal surface degreaser, corrosion inhibition rate >90%.
3. Analytical Chemistry (accounting for 20%
Chromatographic reagents: Separation of bile pigments, fluoride detection;
Microscopic fixative: Tissue decalcification and sample fixation.
Service
* Prompt reply and 24 hours online, professional team to provide best price and high quality product.
* Sample testing support.
* Every batch of products will be tested to ensureits quality.
*The packing also can be according the customers` requirment.
*Any inquiries will be replied within 24 hours.
*we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate. If your markets have any special requirements, let us know.