What is Triphenylphosphine CAS 603-35-0?
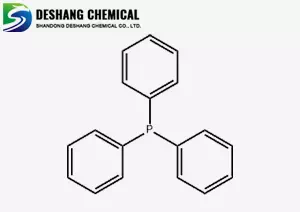
Triphenylphosphine, with the chemical formula C18H15P and a molecular weight of 262.30, is a white to off-white crystalline powder. It is soluble in ether, benzene, chloroform, glacial acetic acid, and slightly soluble in ethanol, but almost insoluble in water. Its melting point is 78°C (5-81.5°C). The boiling point is higher than 360°C (measured under an inert gas atmosphere). Triphenylphosphine is an important ligand for homogeneous catalysts used in petrochemical and fine chemical production. Moreover, new applications are constantly being developed, and the demand is increasing year by year.
The triphenylphosphine molecule has a non-centrosymmetric tetrahedral structure, with a phosphorus atom carrying a pair of lone pairs of electrons occupying one vertex, while the three benzene molecules occupy the other three vertices. It shows a strong tendency to form enantiomeric type crystals. Similar to the Chemicalbook molecule structure, triphenylmethane has been reported to form orthorhombic crystals with a crystallographic space group of P212121, while triphenylphosphine has only been found to form a monoclinic structure with a centrosymmetric space group of P21/a.
The reaction of triphenylphosphine with oxygen in the air to form triphenyl oxophosphine proceeds very slowly: 2PPh3 + O2 → 2OPPh3. Nitrogen-containing compounds react with triphenylphosphine to form imine analogues, such as the StaudChemicalbookinger reaction: PPh3 + PhN3 → PhNPPh3 + N2. Hydrolyzing the product can yield imines: PPh3 + RN3 + H2O → OPPh3 + N2 + RNH2.
Triphenylphosphine CAS 603-35-0 Use
Triphenylphosphine has extensive applications in the chemical industry. It is an important catalyst for the synthesis of fine chemicals. Through technological improvements, it can be better applied to new petroleum processing catalysts; new biocatalytic technologies and catalysts; environmentally friendly new and efficient catalysts; new organic synthesis catalysts; new efficient catalysts for polyolefins; new materials for catalyst carriers and various new catalytic support materials; it can also be used as new functional fine chemicals for specialized papermaking; suitable for protective exploitation and increasing oil recovery rate in new oil fields; new surfactants; high-performance, water-based functional coatings and additives; new textile dyeing and finishing aids; high-performance, environmentally friendly adhesives; new safe and environmentally friendly pigments and dyes; high-performance, environmentally friendly leather chemicals. Triphenylphosphine can also be used in the chemical synthesis of various important chemical products, such as β-carotene, cefprozil, and intermediates for the synthesis of cefdinir.
Triphenylphosphine is a trisubstituted phosphine compound of phosphine. It mainly exhibits reducing and nucleophilic properties. Its application fields are as follows: 1. An important ligand for homogeneous catalysts used in petrochemical and fine chemical production. 2. A basic raw material for rhodium phosphine complex catalysts. 3. Plays an important role in reactions for synthesizing drugs such as vitamin D2, vitamin A, and chloromycetin, as well as plant pigments. 4. A brightening agent, heat stabilizer, light stabilizer, antioxidant, flame retardant, antistatic agent, ozone-resistant agent for rubber, and analytical reagent in the dye industry.
Triphenylphosphine CAS 603-35-0 safety
Category
Toxic
Item toxicity classification Poisoning
Acute toxicity
Oral - LD50 for rats: 700 milligrams per kilogram; Oral - LD50 for mice: 1000 milligrams per kilogram
Stimulating data
Skin - Rabbit 500 mg/24 hours (severe) ; Eyes - Rabbit 500 mg/24 hours (mild)
Flammable hazard characteristics
Toxic phosphorus compounds decompose upon heating
Storage and transportation characteristics
The warehouse is well-ventilated, kept at low temperatures and dry; separated from food raw materials
Storage and transportation of fire extinguishing agents
Carbon dioxide, sand, water, foam
Service
* Prompt reply and 24 hours online, professional team to provide best price and high quality product.
* Sample testing support.
* Every batch of products will be tested to ensureits quality.
*The packing also can be according the customers` requirment.
*Any inquiries will be replied within 24 hours.
*we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate. If your markets have any special requirements, let us know.