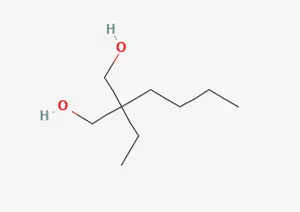
In the field of organic chemistry synthesis, an important compound - 2-butyl-2-ethyl-1, 3-propylene glycol, has a specific reaction pathway and conditions in its synthesis process. This compound is prepared through a series of elaborately designed chemical reaction steps.
Specifically, its synthesis begins with the interaction between formaldehyde and 2-methylpropanal, and in this process, at least one other 2-alkylaldehyde is also required to participate in the reaction. Throughout the entire reaction process, the presence of alkali plays a crucial catalytic role. Under the catalysis of alkali, aldehyde-alcohol condensation reactions occur among these raw materials, which is a crucial reaction step and lays the foundation for subsequent reactions.
After the aldehyde-alcohol condensation reaction is completed, the cross-Cannizzaro reaction will follow immediately. At this reaction stage, the molecular structure undergoes further changes and adjustments, and eventually 2, 2-dialkyl-1, 3-propylene glycol is successfully obtained, including the target product 2-butyl-2-ethyl-1, 3-propylene glycol that we are concerned about.
It is worth noting that the production process of 2-butyl-2-ethyl-1, 3-propylene glycol (BEPD) is not limited to a single route. On the one hand, it can be produced through a salt-free process, which specifically involves first conducting an aldehyde-alcohol addition reaction and then a hydrogenation operation. On the other hand, it can also be prepared through a combination of the hydroxymethylation reaction catalyzed by classic alkali metal hydroxides and the Cannizzaro-type disproportionation reaction of formaldehyde.
In the above-mentioned production process, there is a notable feature, that is, this reaction is highly exothermic and has an extremely fast reaction rate. To ensure that the reaction can proceed safely, stably and effectively, it is necessary to precisely regulate the reaction process by strictly controlling the addition rate of the base catalyst.
In addition, from the perspective of raw materials, the raw material 2-ethylhexal required for the production of 2-butyl-2-ethyl-1, 3-propanediol is itself produced through the aldol condensation reaction and selective hydrogenation steps of n-butyraldehyde. However, compared with the production situation of neopentyl glycol, the production method of this raw material has increased the production cost of BEPD to a certain extent, which has affected the economy and competitiveness of this compound in the market.